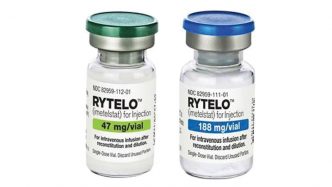
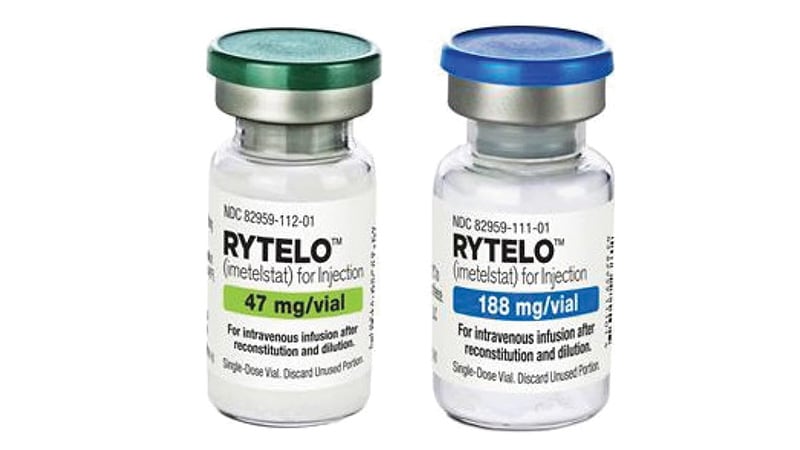
The European Medicines Agency (EMA) has given a positive recommendation for Rytelo (imetelstat) for the treatment of adult patients with transfusion-dependent anemia due to very low-, low-, or intermediate-risk myelodysplastic syndromes.
Myelodysplastic syndromes are a group of cancers in which blood cells in the bone marrow do not mature, build up, and crowd out healthy cells. These are long-term debilitating and life-threatening diseases that feature anemia, leukopenia, and thrombocytopenia, leading to fatigue, recurrent infections, and bleeding disorders. Acute myeloid leukemia develops in about 1 in 3 of those affected.
Anemia is a major clinical concern in myelodysplastic syndromes, with about two-thirds of patients anemic at diagnosis. Eventually, most become transfusion dependent. Recombinant human erythropoietin can produce hematological improvement and improve clinical outcomes, but not all patients respond.
Imetelstat is an antineoplastic agent that blocks the activity of an oligonucleotide telomerase, causing telomere shortening and thereby inhibiting the proliferation of malignant stem and progenitor cells and inducing cell death. This ultimately leads to a reduction in malignant clones.
The EMA’s Committee for Medicinal Products for Human Use (CHMP) recommended granting marketing authorization to Rytelo on the basis of a double-blind controlled study showing that, compared with placebo, the drug reduced the need for red cell transfusions in the first 24 weeks of treatment.
Orphan Drug Designation
Imetelstat was designated as an orphan medicine during its development. This designation, given by the EMA’s Committee for Orphan Medicinal Products, allows a pharmaceutical company to benefit from incentives from the European Union, such as reduced fees and protection from competition once the medicine is placed on the market. It is aimed at medications to be developed for the diagnosis, prevention, or treatment of rare diseases that are life-threatening or very serious. Within the EU, a disease is defined as rare if it affects fewer than 5 in 10,000 people.
At the time of imetelstat’s designation as an orphan drug in 2020, the EMA said that myelodysplastic syndromes affect approximately 2 in 10,000 people in the EU, amounting to a total of around 104,000 people.
The CHMP’s recommendation means that imetelstat is indicated as monotherapy for the treatment of adult patients with transfusion-dependent anemia due to very low-, low-, or intermediate-risk myelodysplastic syndromes without an isolated deletion 5q cytogenetic (non-del 5q) abnormality and who had an unsatisfactory response to or are ineligible for erythropoietin-based therapy.
Hematologists Must Supervise Treatment
The drug will be available as 47 mg and 188 mg powder for concentrating solutions for infusion. Its most common side effects are thrombocytopenia, leukopenia, neutropenia, increased aspartate aminotransferase, increased alanine aminotransferase, increased alkaline phosphatase, asthenia, and headache. Treatment should be administered and monitored under the supervision of physicians and healthcare professionals experienced in hematologic diseases and their treatment.
Detailed recommendations for the use of imetelstat will be published once the European Commission has granted marketing authorization. At this point, the Committee for Orphan Medicinal Products will review the information available to-date to determine if the orphan designation can be maintained. The original designation was based on potential activity, with an assumption that the drug might be of significant benefit for patients with myelodysplastic syndromes. This relied upon early data from the manufacturer suggesting that some patients did not need blood transfusions or needed fewer transfusions after treatment. This assumption needs to be confirmed at the time of marketing authorization in order to maintain the orphan status, which allows the manufacturer 10 years of market exclusivity.
Dr Sheena Meredith is an established medical writer, editor, and consultant in healthcare communications, with extensive experience writing for medical professionals and the general public. She is qualified in medicine and in law and medical ethics.