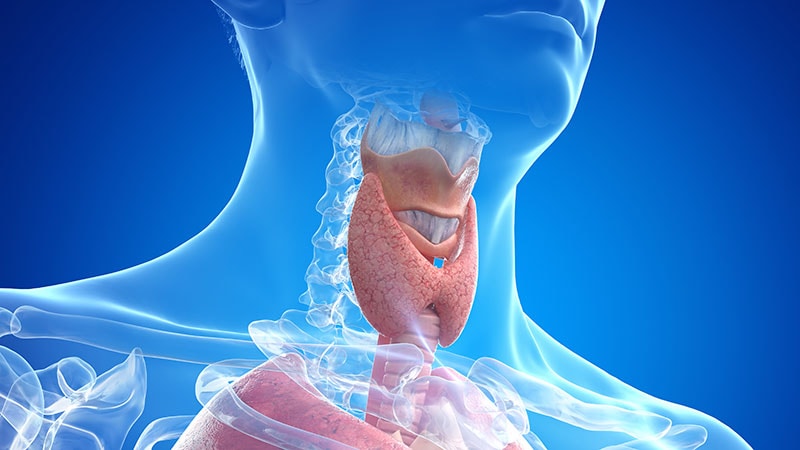
The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended granting marketing authorization for Emcitate (tiratricol) for treating peripheral thyrotoxicosis in patients with Allan-Herndon-Dudley syndrome (AHDS).
Emcitate was designated as an orphan medicinal product for treating AHDS in 2017. The Committee for Orphan Medicinal Products will now assess whether the orphan designations should remain.
AHDS, also known as MCT8 deficiency, is a rare genetic disorder. It is caused by mutations in the gene coding for the thyroid hormone transporter MCT8, which prevent thyroid hormones from entering brain cells. This results in severe intellectual and motor disabilities.
The condition also leads to the accumulation of thyroid hormones in other parts of the body, which can manifest as peripheral thyrotoxicosis and include symptoms like low body weight, tachycardia, and muscle wasting. Complications can include heart failure and death. The prevalence for AHDS is unknown, however it is thought to affect less than one in a million people.
Emcitate’s active ingredient, tiratricol, is an analogue of the thyroid hormone T3. It has a high affinity to thyroid receptors and does not require MCT8 to enter cells. It works similarly to T3 and can restore normal thyroid hormone activity in MCT8-dependent tissues.
The CHMP’s recommendation follows results from a phase 2 trial conducted in children and adults with AHDS in eight European countries and South Africa. Patients were treated with progressively increasing doses of tiratricol for up to 12 months.
The primary endpoint was change in serum T3 concentrations after a year of treatment. Co-primary endpoints included changes in serum thyroid stimulating hormone concentrations, free and total thyroxine (T4), and total reverse T3 from baseline to month 12. Secondary endpoints included changes in body weight, mean heart rate, and systolic blood pressure.
Ultimately, mean serum T3 concentration fell by 63% at month 12. Concentrations of serum thyroid stimulating hormone, serum free and total T4, and reverse T3 concentrations also decreased significantly. All patients saw improvement in at least one secondary end point. While the average number of premature atrial contractions decreased, no improvements were observed for neurodevelopmental delay.
Treatment-related adverse events occurred in 13% of patients and included excessive sweating, anxiety, nightmares, and irritability. Adverse reactions tended to occur at the beginning of treatment or when doses were increased, yet generally subsided during treatment.
Serious adverse events that were not related to the treatment occurred in 39% of patients, including hospital admissions due to infection and one death from pulmonary sepsis leading to multiorgan failure.
Emcitate will be available as 350 µg dispersible tablets. Treatment should be started and monitored by physicians experienced in managing patients with rare genetic disorders such as AHDS.
The CHMP’s opinion on Emcitate will now be sent to the European Commission (EC) for a final decision on the granting of marketing authorization. Detailed recommendations for the drug will be described in the summary of product characteristics, which will be available after the EC has granted it marketing authorization.
Annie Lennon is a medical journalist. Her writing appears on Medscape, Medical News Today, and Psych Central, among other outlets.