—
Mostly expired drugs are no longer effective against circulating SARS-CoV-2 strains
by
Ian Ingram, Managing Editor, MedPage Today
December 13, 2024
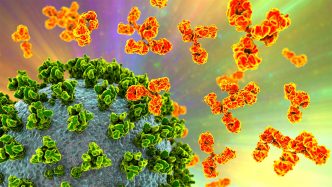
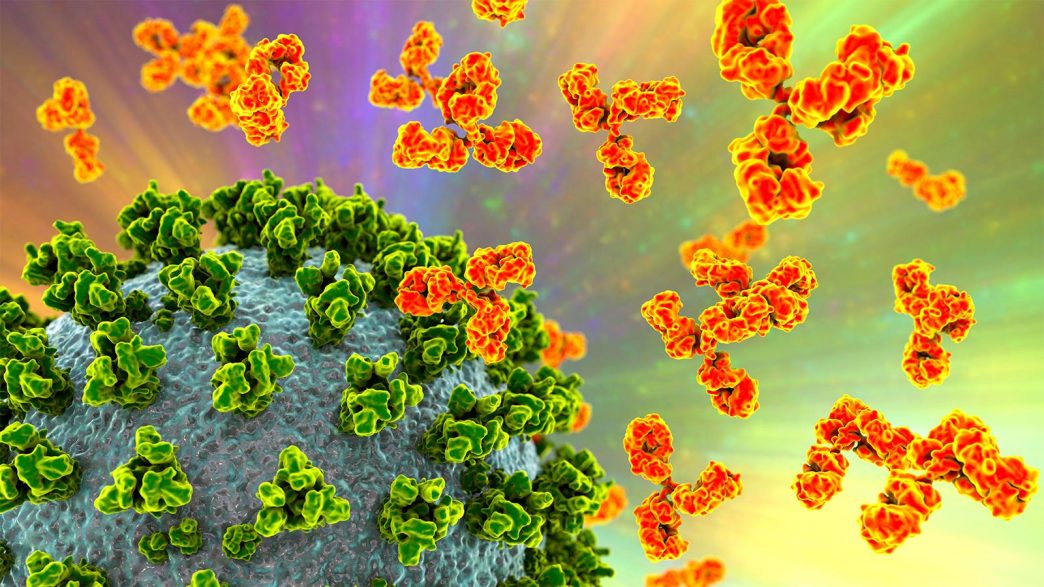
The FDA on Friday revoked the emergency use authorizations (EUAs) of four monoclonal antibodies for COVID-19.
The four drugs — bebtelovimab, sotrovimab, casirivimab/imdevimab (REGN-COV2), and tixagevimab/cilgavimab (Evusheld) — had been authorized for COVID-19 as treatments or as pre- or post-exposure prophylaxis for individuals at high risk of severe outcomes.
Casirivimab/imdevimab had famously been used in 2020 to treat Donald Trump during his bout with COVID-19, while bebtelovimab’s claim to fame may have been the degree of difficulty in pronouncing it.
“The four mAb [monoclonal antibody] products have not been authorized for administration to patients for more than a year due to the high frequency of circulating SARS-CoV-2 variants that are not susceptible to each particular mAb product,” the agency said.
At various points, the FDA had limited each product’s use since their initial authorizations, allowing healthcare facilities to hold on to the drugs in case circumstances changed and they became active against later COVID strains.
“However, the high frequency of circulating SARS-CoV-2 variants that are non-susceptible to these particular mAb products has persisted. In addition, the shelf life for nearly all lots of these products has expired,” the FDA said.
Antivirals such as nirmatrelvir/ritonavir (Paxlovid), remdesivir (Veklury), and molnupiravir (Lagevrio) remain approved or authorized for outpatients at high-risk for severe outcomes of COVID-19.
Currently, pemivibart (Pemgarda) is the only monoclonal antibody authorized for COVID-19, after gaining an EUA earlier this year as pre-exposure prophylaxis in immunocompromised individuals who are unlikely to mount a sufficient immune response following vaccination.
A long-acting monoclonal antibody, pemivibart is specifically authorized for people ages 12 years and older (and weighing 40 kg or more) with moderate-to-severe immune compromise either because of a medical condition or due to immunosuppressant medications. Pemivibart is not for use as post-exposure prophylaxis or in people currently infected with SARS-CoV-2.
The FDA recently expressed concern that newer COVID variants may no longer be susceptible to pemivibart, but quickly reversed course.
According to CDC’s Nowcast tracker, XEC is currently the most common circulating variant of SARS-CoV-2, accounting for an estimated 44% of cases, most closely followed by KP.3.1.1 (39%).
The EUAs for bebtelovimab, sotrovimab, casirivimab/imdevimab, and tixagevimab/cilgavimab were revoked at the request of their respective sponsors, the FDA noted.
-
![author['full_name']](https://clf1.medpagetoday.com/media/images/author/ianIngram_188.jpg)
Ian Ingram is Managing Editor at MedPage Today and helps cover oncology for the site.